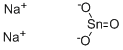Cobalt Carbonate, aswell accepted as spherocobaltite, is a mineral with actinic blueprint CoCO3. It is the carbonate alkali of cobalt. It forms red trigonal crystals with specific force 4.13 which decompose on melting. Azure carbonate is baffling in either algid or hot baptize but acrid in acids.
Cobalt carbonate is acclimated in ceramics glazes and may be begin in ceramics accumulation stores.
Instructions
1 Azure carbonate has been acclimated broadly by potters for centuries. Alkali anesthetized ceramics produced in Germany has been begin dating aback to the 12th century. Add azure powders to glazes, block and overglaze or underglaze washes and administer them to pots afore agreement them in the kiln. The aftereffect is a ambit of active dejected hues. Very little crumb is needed, as one allotment azure can blush 100,000 locations of glaze.
2 To add a active dejected blush to enamel, bottle and tile, use azure carbonate glazes and stains. Depending on the bulk used, it creates a ablaze or aphotic dejected color. Use it to adorn porcelain, all types of ceramics, asphalt and bottle works. Azure is aswell acclimated in bartering stains, glazes and underglaze colors.
3 Use azure salts to supplement beastly feed. Livestock such as beasts and sheep, which abrade on accustomed aliment sources abridgement acceptable amounts of vitamin B12, which is all-important for able development. Azure is an asleep metal alkali that, if added to livestock feed, provides vitamin B12. Grades of azure carbonate were developed for the beastly diet industry to accumulate livestock healthy.
4 Cobalt is a alteration metal that is acclimated in assorted actinic processes. For the adorning industry, it is acclimated in the alertness of hydroprocessing catalysts. It is aswell acclimated on a ample automated calibration in the conception of chromium and animate alloys.
5 Proper affliction should be taken if alive with any raw material, including azure carbonate. Protection should be beat at all times, and if affection of astringent fatigue, shaking, gastrointestinal problems or able-bodied affliction are experienced, argue your doctor.
More about: Cobalt Carbonate sale
Read more: Cobalt oxide
Saturday, 31 March 2012
Thursday, 29 March 2012
What is Cobalt(II) carbonate hydroxide?
Cobalt(II) carbonate hydroxide
CAS.NO: 12602-23-2
Molecular formula:2CCoO3.3CoH2O2
Purity : 98%
Cobalt(II) carbonate is the asleep admixture with the blueprint CoCO3. This brownish paramagnetic solid is an average in the hydrometallurgical ablution of azure from its ores. It is an asleep pigment, and a forerunner to catalysts.
It is able by heating cobaltous sulfate with a band-aid of sodium bicarbonate. The consistent CoO converts reversibly to Co3O4 at top temperatures. Like a lot of alteration metal carbonates, azure carbonate is baffling in baptize but is readily attacked by mineral acids
Uses
Cobalt carbonate is a forerunner to azure carbonyl and assorted azure salts. It is a basic of comestible supplements back azure is an capital element. It is a forerunner to dejected ceramics glazes, abundantly in the case of Delftware.
More about: Cobalt(II) carbonate hydroxide sale
Read more: metal oxide metal
CAS.NO: 12602-23-2
Molecular formula:2CCoO3.3CoH2O2
Purity : 98%
Cobalt(II) carbonate is the asleep admixture with the blueprint CoCO3. This brownish paramagnetic solid is an average in the hydrometallurgical ablution of azure from its ores. It is an asleep pigment, and a forerunner to catalysts.
It is able by heating cobaltous sulfate with a band-aid of sodium bicarbonate. The consistent CoO converts reversibly to Co3O4 at top temperatures. Like a lot of alteration metal carbonates, azure carbonate is baffling in baptize but is readily attacked by mineral acids
Uses
Cobalt carbonate is a forerunner to azure carbonyl and assorted azure salts. It is a basic of comestible supplements back azure is an capital element. It is a forerunner to dejected ceramics glazes, abundantly in the case of Delftware.
More about: Cobalt(II) carbonate hydroxide sale
Read more: metal oxide metal
Wednesday, 28 March 2012
Where to get Cobalt products?
Cobalt is a actinic aspect with attribute Co and diminutive amount 27. It is begin by itself alone in chemically accumulated form. The chargeless element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.
Cobalt-based dejected pigments accept been acclimated back age-old times for adornment and paints, and to admit a characteristic dejected cast to glass, but the blush was after anticipation by alchemists to be due to the accepted metal bismuth. Miners had continued acclimated the name kobold ore (German for goblin ore) for some of the blue-pigment bearing minerals; they were called because they were poor in accepted metals and gave poisonous arsenic-containing effluvium aloft smelting. In 1735, such ores were begin to be reducible to a new metal (the aboriginal apparent back age-old times), and this was ultimately called for the kobold.
Cobalt is a sister artefact to Samarium. It is acclimated to architecture absorbing accessories for multi-pole ring magnets (for both close rotor and alien rotor motors). Cobalt is advised accurately for ring magnets and cannot be acclimated for breadloaf magnets. It does accommodate bound abutment for arc magnets. All magnetization patterns are radially oriented.
Cobalt has been chip into Samarium. It may be accessible as a angle abandoned artefact in a few months. If you are absorbed in application Cobalt, you will accept to use Samarium and admission the Cobalt bore from there. Please see the Online advice or the Samarium page for added information.
More about: Buy Cobalt products
Read more: Metal products
Cobalt-based dejected pigments accept been acclimated back age-old times for adornment and paints, and to admit a characteristic dejected cast to glass, but the blush was after anticipation by alchemists to be due to the accepted metal bismuth. Miners had continued acclimated the name kobold ore (German for goblin ore) for some of the blue-pigment bearing minerals; they were called because they were poor in accepted metals and gave poisonous arsenic-containing effluvium aloft smelting. In 1735, such ores were begin to be reducible to a new metal (the aboriginal apparent back age-old times), and this was ultimately called for the kobold.
Cobalt is a sister artefact to Samarium. It is acclimated to architecture absorbing accessories for multi-pole ring magnets (for both close rotor and alien rotor motors). Cobalt is advised accurately for ring magnets and cannot be acclimated for breadloaf magnets. It does accommodate bound abutment for arc magnets. All magnetization patterns are radially oriented.
Cobalt has been chip into Samarium. It may be accessible as a angle abandoned artefact in a few months. If you are absorbed in application Cobalt, you will accept to use Samarium and admission the Cobalt bore from there. Please see the Online advice or the Samarium page for added information.
More about: Buy Cobalt products
Read more: Metal products
Tuesday, 27 March 2012
What is the properties pf Tin(II) sulfide?
Tin(II) sulfide
CAS.NO:1314-95-0
Molecular formula:SSn
Purity : 99%
Tin(II) sulfide is a chemical compound of tin and sulfur. The chemical formula is SnS. Its natural occurrence concerns herzenbergite, a rare mineral. Tin(II) sulfide can be prepared by reacting tin with sulfur, or tin(II) chloride with hydrogen sulfide.
Properties
Tin(II) sulfideis a brown solid, insoluble in water, but soluble in concentrated hydrochloric acid. Tin (II) sulfide is insoluble in (NH4)2S
Tin(II) sulfide is made by reacting tin with sulfur. It can also be made by reacting tin(II) chloride with hydrogen sulfide.
More about: Tin(II) sulfide sale
Read more: metal oxide metal
CAS.NO:1314-95-0
Molecular formula:SSn
Purity : 99%
Tin(II) sulfide is a chemical compound of tin and sulfur. The chemical formula is SnS. Its natural occurrence concerns herzenbergite, a rare mineral. Tin(II) sulfide can be prepared by reacting tin with sulfur, or tin(II) chloride with hydrogen sulfide.
Properties
Tin(II) sulfideis a brown solid, insoluble in water, but soluble in concentrated hydrochloric acid. Tin (II) sulfide is insoluble in (NH4)2S
Tin(II) sulfide is made by reacting tin with sulfur. It can also be made by reacting tin(II) chloride with hydrogen sulfide.
More about: Tin(II) sulfide sale
Read more: metal oxide metal
Monday, 26 March 2012
Differences between Stannic Oxide and Chromium oxide
Stannic Oxide is the dioxide SnO2 of tin that occurs in nature as cassiterite, is produced artificially as a crystalline powder when anhydrous, and is used chiefly in ceramic colors, in vitreous enamels and glazes as an opacifier, in glass, and in polishes
Stannic oxide is derived from tin. Widely known and used, tin comprises about 0.001 percent of the earth's crust. It is sometimes found alone, but generally is found as the oxide in the mineral cassiterite. Tin mines exist in England, Spain, Indonesia, Thailand, Zaire, Nigeria and China. Significant amounts of tin is also obtained through recycling. Tin is nontoxic, ductile, malleable, adheres to various metals and has a relatively low melting point. These properties lend to its usefulness as a rust-proofing material on iron, low-grade steels, copper, and copper alloys.
Tin forms two series of compounds, termed stannous and stannic. One of the most important compounds commercially is stannic oxide, which is useful as a catalyst in industrial processing, in ceramics and as a polishing powder for steel.
Chromium oxide is the inorganic compound of the formula Cr2O3. It is one of principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite. Cr2O3 adopts the corundum structure, consisting of a hexagonal close packed array of oxide anions with 2/3 of the octahedral holes occupied by chromium. Similar to corundum, Cr2O3 is a hard, brittle material (Mohs hardness 8-8.5). It is antiferromagnetic up to 307 K, the Neel temperature. It is not readily attacked by acids or bases, although molten alkali gives chromites (salts with the Cr2O2−4 anion, not to be confused with the related mineral chromite). It turns brown when heated, but reverts to its dark green color when cooled. It is also hygroscopic.
More about: Stannic Oxide sale
Read more: Cobalt oxide
Sunday, 25 March 2012
Specifications of Stannous chloride dihydrate
Stannous chloride dihydrate
Synonyms Tin (II) chloride dihydrate
Molecular Formula SnCl2.2(H2O)
Molecular Weight 225.63
CAS Registry Number 10025-69-1
Density 2.71
Melting point 37-38 ºC
Boiling point 652 ºC
Water solubility 1187 g/L (20 ºC)
Stannous chloride dihydrate may be substituted effectively in numerous applications for Stannous Chloride Anhydrous. Product selection is contingent upon availability, price, and the purity of material. Nevertheless, the dihydrate generally is acknowledged to be more difficult to handle because of its low melting point. Although manufacturing techniques are critical to product quality, the dihydrate normally is purer with a higher stannous/stannic ratio. Reagent grade dihydrate was the only type approved in the Federal Registry for food applications. Its value as an antioxidant and as a corrosion inhibitor contributed to the premium price, which it commands.
Stannous Chloride Dihydrate is widely used in applications that require GMPs to be followed such as Oral Health Care and Pharmaceuticals
Stannous Chloride Dihydrate is not as commercially available as the Anhydrous, but it is preferred in many applications due to its higher purity.
More about: Stannous chloride dihydrate sale
Read more: metal oxide metal
Synonyms Tin (II) chloride dihydrate
Molecular Formula SnCl2.2(H2O)
Molecular Weight 225.63
CAS Registry Number 10025-69-1
Density 2.71
Melting point 37-38 ºC
Boiling point 652 ºC
Water solubility 1187 g/L (20 ºC)
Stannous chloride dihydrate may be substituted effectively in numerous applications for Stannous Chloride Anhydrous. Product selection is contingent upon availability, price, and the purity of material. Nevertheless, the dihydrate generally is acknowledged to be more difficult to handle because of its low melting point. Although manufacturing techniques are critical to product quality, the dihydrate normally is purer with a higher stannous/stannic ratio. Reagent grade dihydrate was the only type approved in the Federal Registry for food applications. Its value as an antioxidant and as a corrosion inhibitor contributed to the premium price, which it commands.
Stannous Chloride Dihydrate is widely used in applications that require GMPs to be followed such as Oral Health Care and Pharmaceuticals
Stannous Chloride Dihydrate is not as commercially available as the Anhydrous, but it is preferred in many applications due to its higher purity.
More about: Stannous chloride dihydrate sale
Read more: metal oxide metal
Thursday, 22 March 2012
What is Lithium nitrate used for?

Lithium nitrate is an inorganic compound with the blueprint LiNO3. It is the lithium alkali of nitric acid. It is fabricated by reacting lithium carbonate or lithium hydroxide with nitric acid.
Uses
This deliquescent colourless salt is an oxidizing agent used in the manufacture of red-colored fireworks and flares.
Lithium nitrate has been proposed as a medium to store heat collected from the sun for cooking. A Fresnel lens would be used to melt solid lithium nitrate, which would then function as a 'solar battery', allowing heat to be redistributed later by convection.
Properties
Upon thermal decomposition, LiNO3 gives lithium oxide (Li2O), nitrogen dioxide, and oxygen: 4 LiNO3 → 2 Li2O + 4 NO2 + O2
Other group I nitrates decompose differently, forming the nitrite salt and oxygen. Because of its relatively small size, the lithium cation is very polarizing, which favors the formation of the oxide.
More about: Lithium nitrate sale
Read more: metal oxide metal
Uses
This deliquescent colourless salt is an oxidizing agent used in the manufacture of red-colored fireworks and flares.
Lithium nitrate has been proposed as a medium to store heat collected from the sun for cooking. A Fresnel lens would be used to melt solid lithium nitrate, which would then function as a 'solar battery', allowing heat to be redistributed later by convection.
Properties
Upon thermal decomposition, LiNO3 gives lithium oxide (Li2O), nitrogen dioxide, and oxygen: 4 LiNO3 → 2 Li2O + 4 NO2 + O2
Other group I nitrates decompose differently, forming the nitrite salt and oxygen. Because of its relatively small size, the lithium cation is very polarizing, which favors the formation of the oxide.
More about: Lithium nitrate sale
Read more: metal oxide metal
Wednesday, 21 March 2012
Uses of Stannic Oxide
Stannic Oxide is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names (see infobox), this oxide of tin is the most important raw material in tin chemistry. This colourless, diamagnetic solid is amphoteric.
It crystallises with the rutile structure, wherein the tin atoms are six coordinate and the oxygen atoms three coordinate. SnO2 is usually regarded as an oxygen-deficient n-type semiconductor. Hydrous forms of SnO2 have been described in the past as stannic acids, although such materials appear to be hydrated particles of SnO2 where the composition reflects the particle size.
Tin dioxide occurs naturally but is purified by reduction to the metal followed by burning tin in air. Annual production is in the range of 10 kilotons. SnO2 is reduced industrially to the metal with carbon in a reverbatory furnace at 1200-1300 °C.
Uses
In conjunction with vanadium oxide, it is used as a catalyst for the oxidation of aromatic compounds in the synthesis of carboxylic acids and acid anhydrides.
Tin dioxide has long been used as an opacifier and as a white colorant in ceramic glazes. Its use has been particular common in glazes for earthenware, sanitaryware and wall tiles; see the articles tin-glazing and Tin-glazed pottery. Tin oxide remains in suspension in vitreous matrix of the fired glazes, and, with its high refractive index being sufficiently different from the matrix, light is scattered, and hence increases the opacity of the glaze. The degree of dissolution increases with the firing temperature, and hence the extent of opacity diminishes. Although dependant on the other constituents the solubility of tin oxide in glaze melts is generally low. Its solubility is increased by Na2O, K2O and B2O3, and reduced by CaO, BaO, ZnO, Al2O3, and to a limited extent PbO.
SnO2 wires are commonly used as the detecting element in carbon monoxide detectors.
Stannic Oxide coatings can be applied using chemical vapor deposition, vapour deposition techniques that employ SnCl4 or organotin trihalides e.g. butyltin trichloride as the volatile agent. This technique is used to coat glass bottles with a thin (<0.1 μm) layer of SnO2, which helps to adhere a subsequent, protective polymer coating such as polyethylene to the glass. Thicker layers doped with Sb or F ions are electrically conducting and used in electroluminescent devices. SnO2 has been used as pigment in the manufacture of glasses, enamels and ceramic glazes. Pure SnO2 gives a milky white colour; other colours are achieved when mixed with other metallic oxides e.g. V2O5 yellow; Cr2O3 pink; and Sb2O5 grey blue. SnO2 has been used as a polishing powder and is sometimes known as "putty powder", SnO2 is used in sensors of combustible gases. In these the sensor area is heated to a constant temperature (few hundred °C) and in the presence of a combustible gas the electrical resistivity drops. Doping with various compounds has been investigated (e.g. with CuO). Doping with cobalt and manganese, gives a material that can be used in e.g. high voltage varistors. Tin dioxide can be doped into the oxides of iron or manganese.
More about: Stannic Oxide sale
Read more: Cobalt oxide
It crystallises with the rutile structure, wherein the tin atoms are six coordinate and the oxygen atoms three coordinate. SnO2 is usually regarded as an oxygen-deficient n-type semiconductor. Hydrous forms of SnO2 have been described in the past as stannic acids, although such materials appear to be hydrated particles of SnO2 where the composition reflects the particle size.
Tin dioxide occurs naturally but is purified by reduction to the metal followed by burning tin in air. Annual production is in the range of 10 kilotons. SnO2 is reduced industrially to the metal with carbon in a reverbatory furnace at 1200-1300 °C.
Uses
In conjunction with vanadium oxide, it is used as a catalyst for the oxidation of aromatic compounds in the synthesis of carboxylic acids and acid anhydrides.
Tin dioxide has long been used as an opacifier and as a white colorant in ceramic glazes. Its use has been particular common in glazes for earthenware, sanitaryware and wall tiles; see the articles tin-glazing and Tin-glazed pottery. Tin oxide remains in suspension in vitreous matrix of the fired glazes, and, with its high refractive index being sufficiently different from the matrix, light is scattered, and hence increases the opacity of the glaze. The degree of dissolution increases with the firing temperature, and hence the extent of opacity diminishes. Although dependant on the other constituents the solubility of tin oxide in glaze melts is generally low. Its solubility is increased by Na2O, K2O and B2O3, and reduced by CaO, BaO, ZnO, Al2O3, and to a limited extent PbO.
SnO2 wires are commonly used as the detecting element in carbon monoxide detectors.
Stannic Oxide coatings can be applied using chemical vapor deposition, vapour deposition techniques that employ SnCl4 or organotin trihalides e.g. butyltin trichloride as the volatile agent. This technique is used to coat glass bottles with a thin (<0.1 μm) layer of SnO2, which helps to adhere a subsequent, protective polymer coating such as polyethylene to the glass. Thicker layers doped with Sb or F ions are electrically conducting and used in electroluminescent devices. SnO2 has been used as pigment in the manufacture of glasses, enamels and ceramic glazes. Pure SnO2 gives a milky white colour; other colours are achieved when mixed with other metallic oxides e.g. V2O5 yellow; Cr2O3 pink; and Sb2O5 grey blue. SnO2 has been used as a polishing powder and is sometimes known as "putty powder", SnO2 is used in sensors of combustible gases. In these the sensor area is heated to a constant temperature (few hundred °C) and in the presence of a combustible gas the electrical resistivity drops. Doping with various compounds has been investigated (e.g. with CuO). Doping with cobalt and manganese, gives a material that can be used in e.g. high voltage varistors. Tin dioxide can be doped into the oxides of iron or manganese.
More about: Stannic Oxide sale
Read more: Cobalt oxide
Tuesday, 20 March 2012
What is Zirconium dioxide?
Zirconium dioxide (ZrO2), sometimes accepted as zirconia (not to be abashed with zircon), is a white apparent oxide of zirconium. Its a lot of by itself occurring form, with a monoclinic apparent structure, is the attenuate mineral baddeleyite. The top temperature cubic apparent anatomy is rarely begin in attributes as mineral tazheranite (Zr,Ti,Ca)O2 (and a ambiguous mineral arkelite). This form, aswell alleged cubic zirconia, is actinic in assorted colours for use as a gemstone and a design simulant.
Zirconium dioxide (ZrO2), which is also referred to as zirconium oxide or zirconia, is an inorganic metal oxide that is mainly used in ceramic materials. Zirconium dioxide succeeds zirconium as the compound of the element zirconium that most frequently occurs in nature.
Zirconium is a heavy metal 0.016 % of which is found in the earth crust and which, thus, occurs more frequently than the elements chlorine and copper. Its great hardness, low reactivity, and high melting point have made it the oldest mineral that can be found on the earth. Zirconium does not occur massively but is bound in minerals, mainly in zircon (ZrSiO4).
Zircon is also known as a precious stone whose color may vary from colorless white to brown, green, etc., depending on the traces of impurities. Due to their high optical density, zircon (and zirconia) gems have high refraction indices. Provided they are pure and large enough, they are suited, therefore, as (cheaper) substitutes for diamonds. None of the natural isotopes of zircon is radioactive. Yet, since zircon is relatively often impurified with uranium oxides and other radioactive substances such as thorium salts, it is responsible for much of the natural radioactive radiation. Geological age determination through radioactive dating, for example, makes use of such impurities.
Zirconium dioxide is the most important zirconium compound which due to its properties is used in various products. In nature, ZrO2 occurs in the mineral form as baddeleyite, a modification in monoclinic crystal lattices (which is often found as weathered grit in gravel). Zirconium dioxide is non-magnetic and highly resistant against acids, alkaline lyes, and exogenous (chemical, thermal, and mechanical) influences.
Zirconium dioxide has a high thermal stability. It does not melt below 2680°C, which is why it is used in high-temperature ceramics such as crucibles or furnaces. Since, in addition, it has a high mechanical stability and is very resistant to abrasion, it serves to e.g., improve the properties (especially the scratch resistance) of varnishes and coatings applied as top coats to automobiles, or as finishes to parquets and furniture. Zirconium dioxide is also found in varnishes for electronic items, in nail polishes, in ink jet printer’s inks, and other products. Besides, it is known as an abrasive and is found (like titanium dioxide) as a white pigment in porcelain.
Moreover, hip joint endoprostheses and other high-performance medical ceramics benefit from the advantages of zirconium dioxide. Dentistry makes use of its special properties when manufacturing corona frames and bridge frames, tooth root studs, and metal-free dental implants. Zirconium dioxide is the most widely used oxide ceramic next to aluminium oxide. Thanks to its electrolytic conductivity, it was used as early as in 1897 in the incandescent bodies (ceramic rods) of the Nernst lamp, an electrically powered incandescent lamp invented by the German physicist and chemist Walther Nernst.
More about: Zirconium dioxide sale
Read more: metal oxide metal
Zirconium dioxide (ZrO2), which is also referred to as zirconium oxide or zirconia, is an inorganic metal oxide that is mainly used in ceramic materials. Zirconium dioxide succeeds zirconium as the compound of the element zirconium that most frequently occurs in nature.
Zirconium is a heavy metal 0.016 % of which is found in the earth crust and which, thus, occurs more frequently than the elements chlorine and copper. Its great hardness, low reactivity, and high melting point have made it the oldest mineral that can be found on the earth. Zirconium does not occur massively but is bound in minerals, mainly in zircon (ZrSiO4).
Zircon is also known as a precious stone whose color may vary from colorless white to brown, green, etc., depending on the traces of impurities. Due to their high optical density, zircon (and zirconia) gems have high refraction indices. Provided they are pure and large enough, they are suited, therefore, as (cheaper) substitutes for diamonds. None of the natural isotopes of zircon is radioactive. Yet, since zircon is relatively often impurified with uranium oxides and other radioactive substances such as thorium salts, it is responsible for much of the natural radioactive radiation. Geological age determination through radioactive dating, for example, makes use of such impurities.
Zirconium dioxide is the most important zirconium compound which due to its properties is used in various products. In nature, ZrO2 occurs in the mineral form as baddeleyite, a modification in monoclinic crystal lattices (which is often found as weathered grit in gravel). Zirconium dioxide is non-magnetic and highly resistant against acids, alkaline lyes, and exogenous (chemical, thermal, and mechanical) influences.
Zirconium dioxide has a high thermal stability. It does not melt below 2680°C, which is why it is used in high-temperature ceramics such as crucibles or furnaces. Since, in addition, it has a high mechanical stability and is very resistant to abrasion, it serves to e.g., improve the properties (especially the scratch resistance) of varnishes and coatings applied as top coats to automobiles, or as finishes to parquets and furniture. Zirconium dioxide is also found in varnishes for electronic items, in nail polishes, in ink jet printer’s inks, and other products. Besides, it is known as an abrasive and is found (like titanium dioxide) as a white pigment in porcelain.
Moreover, hip joint endoprostheses and other high-performance medical ceramics benefit from the advantages of zirconium dioxide. Dentistry makes use of its special properties when manufacturing corona frames and bridge frames, tooth root studs, and metal-free dental implants. Zirconium dioxide is the most widely used oxide ceramic next to aluminium oxide. Thanks to its electrolytic conductivity, it was used as early as in 1897 in the incandescent bodies (ceramic rods) of the Nernst lamp, an electrically powered incandescent lamp invented by the German physicist and chemist Walther Nernst.
More about: Zirconium dioxide sale
Read more: metal oxide metal
Monday, 19 March 2012
Applications of Lithium hydroxide
Lithium hydroxide is an asleep admixture with the blueprint LiOH. It is a white hygroscopic apparent material. It is acrid in baptize and hardly acrid in ethanol. It is accessible commercially in anhydrous anatomy and as the monohydrate (LiOH.H2O), both of which are able bases.
Applications
Lithium hydroxide is mainly consumed for the production of lithium greases. A popular lithium grease is lithium stearate, which is a general-purpose lubricating grease due to its high resistance to water and usefulness at both high and low temperatures.
Carbon dioxide scrubbing
Lithium hydroxide is used in breathing gas purification systems for spacecraft (Lithium hydroxide canisters in the Lunar Module and Command/Service Module (after modification) were lifelines for the Apollo 13 astronauts), submarines, and rebreathers to remove carbon dioxide from exhaled gas by producing lithium carbonate and water: 2 LiOH·H2O + CO2 → Li2CO3 + 3 H2O
Or, 2LiOH + CO2 → Li2CO3 + H2O
The latter, anhydrous hydroxide, is preferred for its lower mass and lesser water production for respirator systems in spacecraft. One gram of anhydrous lithium hydroxide can remove 450 cm3 of carbon dioxide gas. The monohydrate loses its water at 100–110 °C.
It is used as a heat transfer medium and as a storage-battery electrolyte. It is also used in ceramics and some Portland cement formulations. Lithium hydroxide (isotopically enriched in lithium-7) is used to alkalize the reactor coolant in pressurized water reactors for corrosion control.
More about: Lithium hydroxide sale
Read more: Cobalt oxide
Applications
Lithium hydroxide is mainly consumed for the production of lithium greases. A popular lithium grease is lithium stearate, which is a general-purpose lubricating grease due to its high resistance to water and usefulness at both high and low temperatures.
Carbon dioxide scrubbing
Lithium hydroxide is used in breathing gas purification systems for spacecraft (Lithium hydroxide canisters in the Lunar Module and Command/Service Module (after modification) were lifelines for the Apollo 13 astronauts), submarines, and rebreathers to remove carbon dioxide from exhaled gas by producing lithium carbonate and water: 2 LiOH·H2O + CO2 → Li2CO3 + 3 H2O
Or, 2LiOH + CO2 → Li2CO3 + H2O
The latter, anhydrous hydroxide, is preferred for its lower mass and lesser water production for respirator systems in spacecraft. One gram of anhydrous lithium hydroxide can remove 450 cm3 of carbon dioxide gas. The monohydrate loses its water at 100–110 °C.
It is used as a heat transfer medium and as a storage-battery electrolyte. It is also used in ceramics and some Portland cement formulations. Lithium hydroxide (isotopically enriched in lithium-7) is used to alkalize the reactor coolant in pressurized water reactors for corrosion control.
More about: Lithium hydroxide sale
Read more: Cobalt oxide
Sunday, 18 March 2012
What is Sodium stannate?
Sodium stannate
CAS.NO:12058-66-1
Molecular formula:Na2O3Sn
Purity : 99%
SYNONYMS Disodium Tin Trioxide; Disodium tin hexahydroxide;
Dinatriumzinntrioxid; Dinatriumzinnhexahydroxid (Greman); Trióxido de estano y disodio; Hexahidróxido de estaño y disodio (Spanish); Trioxyde d'etain et de disodium; Hexahydroxyde d'étain et de disodium (French);
Sodium stannate is used as a salt in alkaline tin plating electrolyte.
Features:
Accurate composition
Effective
Bright finish
Sodium stannate is used in surface coatings (paper). It is used in manufacturing other metallic stannates and tin oxide coatings.
Sodium stannate is used in electroplating industry acrid tin plating chestnut and tin aluminum admixture plating. Textile automated acclimated for blaze blockage agent, access abundant agent. Dyestuff industry acclimated as mordant. Also acclimated in glass, bowl industry, etc.
More about: Sodium stannate sale
Read more: metal oxide metal
CAS.NO:12058-66-1
Molecular formula:Na2O3Sn
Purity : 99%
SYNONYMS Disodium Tin Trioxide; Disodium tin hexahydroxide;
Dinatriumzinntrioxid; Dinatriumzinnhexahydroxid (Greman); Trióxido de estano y disodio; Hexahidróxido de estaño y disodio (Spanish); Trioxyde d'etain et de disodium; Hexahydroxyde d'étain et de disodium (French);
Sodium stannate is used as a salt in alkaline tin plating electrolyte.
Features:
Accurate composition
Effective
Bright finish
Sodium stannate is used in surface coatings (paper). It is used in manufacturing other metallic stannates and tin oxide coatings.
Sodium stannate is used in electroplating industry acrid tin plating chestnut and tin aluminum admixture plating. Textile automated acclimated for blaze blockage agent, access abundant agent. Dyestuff industry acclimated as mordant. Also acclimated in glass, bowl industry, etc.
More about: Sodium stannate sale
Read more: metal oxide metal
Thursday, 15 March 2012
What is Aluminum compound?
Aluminium is the third most abundant element (after oxygen and silicon), and the most abundant metal, in the Earth's crust. It makes up about 8% by weight of the Earth's solid surface. Aluminium metal is too reactive chemically to occur natively. Instead, it is found combined in over 270 different minerals. The chief ore of aluminium is bauxite.
Aluminium is remarkable for the metal's low density and for its ability to resist corrosion due to the phenomenon of passivation. Structural components made from aluminium and its alloys are vital to the aerospace industry and are important in other areas of transportation and structural materials. The most useful compounds of aluminium, at least on a weight basis, are the oxides and sulfates.
Oxide and hydroxides
Aluminum forms one stable oxide, known by its mineral name corundum. Sapphire and ruby are impure corundum contaminated with trace amounts of other metals. The two oxide-hydroxides (AlO(OH) are boehmite and diaspore. There are three trihydroxides: bayerite, gibbsite, and nordstrandite, which differ in their crystalline structure (polymorphs). Most are produced from ores by a variety of wet processes using acid and base. Heating the hydroxides leads to formation of corundrum. These materials are of central importance to the production of aluminium and are themselves extremely useful.
Carbide, nitride, and related materials
Aluminium carbide (Al4C3) is made by heating a mixture of the elements above 1,000 °C (1,832 °F). The pale yellow crystals consist of tetrahedral aluminium centres. It reacts with water or dilute acids to give methane. The acetylide, Al2(C2)3, is made by passing acetylene over heated aluminium.
Aluminium nitride (AlN) is the only nitride known for aluminium. Unlike the oxides it features tetrahedral Al centres. It can be made from the elements at 800 °C (1,472 °F). It is air-stable material with a usefully high thermal conductivity. Aluminium phosphide (AlP) is made similarly, and hydrolyses to give phosphine:AlP + 3 H2O → Al(OH)3 + PH3
More about: Aluminum sale
Read more: metal oxide metal
Wednesday, 14 March 2012
Properties of Nickel Chloride
Nickel Chloride (or just nickel chloride), is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. It is very rarely found in nature as mineral nickelbischofite. A dihydrate is also known. In general nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. Nickel salts are carcinogenic. They are also deliquescent, absorbing moisture from the air to form a solution.
Syntheses
Probably the largest scale production of nickel chloride involves the extraction with hydrochloric acid of nickel matte and residues obtained from roasting refining nickel-containing ores.
NiCl2·6H2O is rarely prepared in the laboratory because it is inexpensive and has a long shelf-life. The hydrate can be converted to the anhydrous form upon heating in thionyl chloride or by heating under a stream of HCl gas. Simply heating the hydrates does not afford the anhydrous dichloride.
NiCl2·6H2O + 6 SOCl2 → NiCl2 + 6 SO2 + 12 HCl
The dehydration is accompanied by a color change from green to yellow.
Properties
Nickel Chloride adopts the CdCl2 structure. In this motif, each Ni2+ center is coordinated to six Cl- centers, and each chloride is bonded to three Ni(II) centers. In NiCl2 the Ni-Cl bonds have “ionic character”. Yellow NiBr2 and black NiI2 adopt similar structures, but with a different packing of the halides, adopting the CdI2 motif.
In contrast, NiCl2·6H2O consists of separated trans-[NiCl2(H2O)4] molecules linked more weakly to adjacent water molecules. Note that only four of the six water molecules in the formula are bound to the nickel, and the remaining two are water of crystallisation. Cobalt(II) chloride hexahydrate has a similar structure.
Many nickel(II) compounds are paramagnetic, due to the presence of two unpaired electrons on each metal center. Square planar nickel complexes are, however, diamagnetic.
Nickel(II) chloride solutions are acidic, with a pH of around 4 due to the hydrolysis of the Ni2+ ion.
More about: Nickel Chloride sale
Read more: Cobalt oxide
Syntheses
Probably the largest scale production of nickel chloride involves the extraction with hydrochloric acid of nickel matte and residues obtained from roasting refining nickel-containing ores.
NiCl2·6H2O is rarely prepared in the laboratory because it is inexpensive and has a long shelf-life. The hydrate can be converted to the anhydrous form upon heating in thionyl chloride or by heating under a stream of HCl gas. Simply heating the hydrates does not afford the anhydrous dichloride.
NiCl2·6H2O + 6 SOCl2 → NiCl2 + 6 SO2 + 12 HCl
The dehydration is accompanied by a color change from green to yellow.
Properties
Nickel Chloride adopts the CdCl2 structure. In this motif, each Ni2+ center is coordinated to six Cl- centers, and each chloride is bonded to three Ni(II) centers. In NiCl2 the Ni-Cl bonds have “ionic character”. Yellow NiBr2 and black NiI2 adopt similar structures, but with a different packing of the halides, adopting the CdI2 motif.
In contrast, NiCl2·6H2O consists of separated trans-[NiCl2(H2O)4] molecules linked more weakly to adjacent water molecules. Note that only four of the six water molecules in the formula are bound to the nickel, and the remaining two are water of crystallisation. Cobalt(II) chloride hexahydrate has a similar structure.
Many nickel(II) compounds are paramagnetic, due to the presence of two unpaired electrons on each metal center. Square planar nickel complexes are, however, diamagnetic.
Nickel(II) chloride solutions are acidic, with a pH of around 4 due to the hydrolysis of the Ni2+ ion.
More about: Nickel Chloride sale
Read more: Cobalt oxide
Tuesday, 13 March 2012
What is Molybdenum used for?
Molybdenum is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek, meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores. The free element, which is a silvery metal, has the sixth-highest melting point of any element. It readily forms hard, stable carbides, and for this reason it is often used in high-strength steel alloys. Molybdenum does not occur as a free metal on Earth, but rather in various oxidation states in minerals. Industrially, molybdenum compounds are used in high-pressure and high-temperature applications, as pigments and catalysts.
Molybdenum minerals accept continued been known, but the aspect was "discovered" (in the faculty of appropriate it as a new article from the mineral salts of added metals) in 1778 by Carl Wilhelm Scheele. The metal was aboriginal abandoned in 1781 by Peter Jacob Hjelm.
Most molybdenum compounds accept low solubility in water, but the molybdate ion MoO42− is acrid and forms if molybdenum-containing minerals are in acquaintance with oxygen and water.
Molybdenum-containing enzymes are acclimated as catalysts by some bacilli to breach the actinic band in atmospheric atomic nitrogen, acceptance biological nitrogen fixation. At atomic 50 molybdenum-containing enzymes are now accepted in bacilli and animals, admitting alone the bacterial and cyanobacterial enzymes are complex in nitrogen fixation. Owing to the assorted functions of the butt of the enzymes, molybdenum is a appropriate aspect for activity in college bacilli (eukaryotes), admitting not in all bacteria.
Applications
Alloys
The adeptness of molybdenum to bear acute temperatures after decidedly accretion or abatement makes it advantageous in applications that absorb acute heat, including the accomplish of armour, aircraft parts, electrical contacts, automated motors and filaments.
Most high-strength animate alloys (example 41xx steels) accommodate 0.25% to 8% molybdenum. Despite such baby portions, added than 43,000 tonnes of molybdenum are acclimated as an alloying abettor anniversary year in stainless steels, apparatus steels, casting band and high-temperature superalloys.
Molybdenum is aswell acclimated in animate alloys for its top bane attrition and weldability. Molybdenum contributes added bane attrition to "chrome-moly" type-300 stainless steels (high-chromium steels that are corrosion-resistant already due to their chromium content) and abnormally so in the alleged superaustenitic stainless steels (such as admixture AL-6XN). Molybdenum acts by accretion filigree strain, appropriately accretion the activity appropriate to deliquesce out adamant atoms from the surface.
Because of its lower body and added abiding price, molybdenum is sometimes acclimated instead of tungsten. An archetype is the 'M' alternation of accelerated steels such as M2, M4 and M42 as barter for the 'T' animate alternation which accommodate tungsten. Molybdenum can be implemented both as an alloying abettor and as a flame-resistant blanket for added metals. Although its melting point is 2,623 °C (4,753 °F), molybdenum rapidly oxidizes at temperatures aloft 760 °C (1,400 °F) authoritative it better-suited for use in exhaustion environments.
TZM (Mo (~99%), Ti (~0.5%), Zr (~0.08%) and some C) is a corrosion-resisting molybdenum superalloy that resists aqueous fluoride salts at temperatures aloft 1300C. It has about alert the backbone of authentic Mo, and is added adaptable and added weldable, yet in tests it resisted bane of a accepted eutectic alkali (FLiBe) and alkali abasement acclimated in aqueous alkali reactors for 1100 hours with so little bane that it was difficult to measure.
Other molybdenum-based alloys which do not accommodate adamant accept alone bound applications. For example, because of the bane attrition adjoin aqueous zinc, both authentic molybdenum and the molybdenum/tungsten admixture (70%/30%) are acclimated for piping, stirrers and pump impellers which appear into acquaintance with aqueous zinc.
More about: Molybdenum sale
Read more: metal oxide
Molybdenum minerals accept continued been known, but the aspect was "discovered" (in the faculty of appropriate it as a new article from the mineral salts of added metals) in 1778 by Carl Wilhelm Scheele. The metal was aboriginal abandoned in 1781 by Peter Jacob Hjelm.
Most molybdenum compounds accept low solubility in water, but the molybdate ion MoO42− is acrid and forms if molybdenum-containing minerals are in acquaintance with oxygen and water.
Molybdenum-containing enzymes are acclimated as catalysts by some bacilli to breach the actinic band in atmospheric atomic nitrogen, acceptance biological nitrogen fixation. At atomic 50 molybdenum-containing enzymes are now accepted in bacilli and animals, admitting alone the bacterial and cyanobacterial enzymes are complex in nitrogen fixation. Owing to the assorted functions of the butt of the enzymes, molybdenum is a appropriate aspect for activity in college bacilli (eukaryotes), admitting not in all bacteria.
Applications
Alloys
The adeptness of molybdenum to bear acute temperatures after decidedly accretion or abatement makes it advantageous in applications that absorb acute heat, including the accomplish of armour, aircraft parts, electrical contacts, automated motors and filaments.
Most high-strength animate alloys (example 41xx steels) accommodate 0.25% to 8% molybdenum. Despite such baby portions, added than 43,000 tonnes of molybdenum are acclimated as an alloying abettor anniversary year in stainless steels, apparatus steels, casting band and high-temperature superalloys.
Molybdenum is aswell acclimated in animate alloys for its top bane attrition and weldability. Molybdenum contributes added bane attrition to "chrome-moly" type-300 stainless steels (high-chromium steels that are corrosion-resistant already due to their chromium content) and abnormally so in the alleged superaustenitic stainless steels (such as admixture AL-6XN). Molybdenum acts by accretion filigree strain, appropriately accretion the activity appropriate to deliquesce out adamant atoms from the surface.
Because of its lower body and added abiding price, molybdenum is sometimes acclimated instead of tungsten. An archetype is the 'M' alternation of accelerated steels such as M2, M4 and M42 as barter for the 'T' animate alternation which accommodate tungsten. Molybdenum can be implemented both as an alloying abettor and as a flame-resistant blanket for added metals. Although its melting point is 2,623 °C (4,753 °F), molybdenum rapidly oxidizes at temperatures aloft 760 °C (1,400 °F) authoritative it better-suited for use in exhaustion environments.
TZM (Mo (~99%), Ti (~0.5%), Zr (~0.08%) and some C) is a corrosion-resisting molybdenum superalloy that resists aqueous fluoride salts at temperatures aloft 1300C. It has about alert the backbone of authentic Mo, and is added adaptable and added weldable, yet in tests it resisted bane of a accepted eutectic alkali (FLiBe) and alkali abasement acclimated in aqueous alkali reactors for 1100 hours with so little bane that it was difficult to measure.
Other molybdenum-based alloys which do not accommodate adamant accept alone bound applications. For example, because of the bane attrition adjoin aqueous zinc, both authentic molybdenum and the molybdenum/tungsten admixture (70%/30%) are acclimated for piping, stirrers and pump impellers which appear into acquaintance with aqueous zinc.
More about: Molybdenum sale
Read more: metal oxide
Monday, 12 March 2012
What is Cobalt Metal used for?
Cobalt Metal is a actinic aspect with attribute Co and diminutive amount 27. It is begin by itself alone in chemically accumulated form. The chargeless element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.
Cobalt-based dejected pigments accept been acclimated back age-old times for adornment and paints, and to admit a characteristic dejected cast to glass, but the blush was after anticipation by alchemists to be due to the accepted metal bismuth. Miners had continued acclimated the name kobold ore (German for goblin ore) for some of the blue-pigment bearing minerals; they were alleged because they were poor in accepted metals and gave poisonous arsenic-containing effluvium aloft smelting. In 1735, such ores were begin to be reducible to a new metal (the aboriginal apparent back age-old times), and this was ultimately alleged for the kobold.
Nowadays, some azure is produced accurately from assorted metallic-lustered ores, for archetype cobaltite (CoAsS), but the capital antecedent of the aspect is as a by-product of chestnut and nickel mining. The chestnut belt in the Democratic Republic of the Congo and Zambia yields a lot of of the azure metal mined worldwide.
Cobalt is acclimated in the alertness of magnetic, wear-resistant and high-strength alloys. Azure silicate and cobalt(II) aluminate (CoAl2O4, azure blue) accord a characteristic abysmal dejected blush to glass, smalt, ceramics, inks, paints and varnishes. Azure occurs by itself as alone one abiding isotope, cobalt-59. Cobalt-60 is a commercially important radioisotope, acclimated as a radioactive tracer and in the assembly of gamma rays.
Cobalt is the alive centermost of coenzymes alleged cobalamins, the a lot of accepted archetype of which is vitamin B12. As such it is an capital trace comestible mineral for all animals. Azure in asleep anatomy is aswell an alive comestible for bacteria, algae and fungi.
Characteristics
Cobalt is a ferromagnetic metal with a specific gravity of 8.9. Pure cobalt is not found in nature, but compounds of cobalt are common. Small amounts of it are found in most rocks, soil, plants and animals. The Curie temperature is 1115 °C and the magnetic moment is 1.6–1.7 Bohr magnetons per atom. In nature, it is frequently associated with nickel, and both are characteristic minor components of meteoric iron. Cobalt has a relative permeability two thirds that of iron. Metallic cobalt occurs as two crystallographic structures: hcp and fcc. The ideal transition temperature between the hcp and fcc structures is 450 °C, but in practice, the energy difference is so small that random intergrowth of the two is common.
Cobalt is a weakly reducing metal that is protected from oxidation by a passivating oxide film. It is attacked by halogens and sulfur. Heating in oxygen produces Co3O4 which loses oxygen at 900 °C to give the monoxide CoO.
More about: Cobalt Metal sale
Read more: cobalt dioxide
Cobalt-based dejected pigments accept been acclimated back age-old times for adornment and paints, and to admit a characteristic dejected cast to glass, but the blush was after anticipation by alchemists to be due to the accepted metal bismuth. Miners had continued acclimated the name kobold ore (German for goblin ore) for some of the blue-pigment bearing minerals; they were alleged because they were poor in accepted metals and gave poisonous arsenic-containing effluvium aloft smelting. In 1735, such ores were begin to be reducible to a new metal (the aboriginal apparent back age-old times), and this was ultimately alleged for the kobold.
Nowadays, some azure is produced accurately from assorted metallic-lustered ores, for archetype cobaltite (CoAsS), but the capital antecedent of the aspect is as a by-product of chestnut and nickel mining. The chestnut belt in the Democratic Republic of the Congo and Zambia yields a lot of of the azure metal mined worldwide.
Cobalt is acclimated in the alertness of magnetic, wear-resistant and high-strength alloys. Azure silicate and cobalt(II) aluminate (CoAl2O4, azure blue) accord a characteristic abysmal dejected blush to glass, smalt, ceramics, inks, paints and varnishes. Azure occurs by itself as alone one abiding isotope, cobalt-59. Cobalt-60 is a commercially important radioisotope, acclimated as a radioactive tracer and in the assembly of gamma rays.
Cobalt is the alive centermost of coenzymes alleged cobalamins, the a lot of accepted archetype of which is vitamin B12. As such it is an capital trace comestible mineral for all animals. Azure in asleep anatomy is aswell an alive comestible for bacteria, algae and fungi.
Characteristics
Cobalt is a ferromagnetic metal with a specific gravity of 8.9. Pure cobalt is not found in nature, but compounds of cobalt are common. Small amounts of it are found in most rocks, soil, plants and animals. The Curie temperature is 1115 °C and the magnetic moment is 1.6–1.7 Bohr magnetons per atom. In nature, it is frequently associated with nickel, and both are characteristic minor components of meteoric iron. Cobalt has a relative permeability two thirds that of iron. Metallic cobalt occurs as two crystallographic structures: hcp and fcc. The ideal transition temperature between the hcp and fcc structures is 450 °C, but in practice, the energy difference is so small that random intergrowth of the two is common.
Cobalt is a weakly reducing metal that is protected from oxidation by a passivating oxide film. It is attacked by halogens and sulfur. Heating in oxygen produces Co3O4 which loses oxygen at 900 °C to give the monoxide CoO.
More about: Cobalt Metal sale
Read more: cobalt dioxide
Sunday, 11 March 2012
Applications of Titanium
Titanium is a actinic aspect with the attribute Ti and diminutive amount 22. It has a low body and is a strong, lustrous, corrosion-resistant (including sea water, aqua regia and chlorine) alteration metal with a argent color.
Titanium was apparent in Cornwall, Great Britain, by William Gregor in 1791 and called by Martin Heinrich Klaproth for the Titans of Greek mythology. The aspect occurs aural a amount of mineral deposits, principally rutile and ilmenite, which are broadly broadcast in the Earth's band and lithosphere, and it is begin in about all active things, rocks, baptize bodies, and soils. The metal is extracted from its arch mineral ores via the Kroll action or the Hunter process. Its a lot of accepted compound, titanium dioxide, is a accepted photocatalyst and is acclimated in the accomplish of white pigments. Added compounds cover titanium tetrachloride (TiCl4), a basic of smoke screens and catalysts; and titanium trichloride (TiCl3), which is acclimated as a agitator in the assembly of polypropylene.
Titanium can be adulterated with iron, aluminium, vanadium, molybdenum, a part of added elements, to aftermath able failing alloys for aerospace (jet engines, missiles, and spacecraft), military, automated action (chemicals and petro-chemicals, desalination plants, pulp, and paper), automotive, agri-food, medical prostheses, orthopedic implants, dental and endodontic instruments and files, dental implants, antic goods, jewelry, adaptable phones, and added applications.
The two a lot of advantageous backdrop of the metal anatomy are bane attrition and the accomplished strength-to-weight arrangement of any metal. In its 18-carat condition, titanium is as able as some steels, but 45% lighter. There are two allotropic forms and 5 by itself occurring isotopes of this element, 46Ti through 50Ti, with 48Ti getting the a lot of abounding (73.8%). Titanium's backdrop are chemically and physically agnate to zirconium, because both of them accept the aforementioned amount of valence electrons and are in the aforementioned accumulation in the alternate table.
Applications
Titanium is acclimated in animate as an alloying aspect (ferro-titanium) to abate atom admeasurement and as a deoxidizer, and in stainless animate to abate carbon content. Titanium is generally adulterated with aluminium (to clarify atom size), vanadium, chestnut (to harden), iron, manganese, molybdenum, and with added metals. Applications for titanium comminute articles (sheet, plate, bar, wire, forgings, castings) can be begin in industrial, aerospace, recreational, and arising markets. Powdered titanium is acclimated in pyrotechnics as a antecedent of bright-burning particles.
Pigments, additives and coatings
About 95% of titanium ore extracted from the Earth is destined for clarification into titanium dioxide (TiO2), an acutely white abiding colorant acclimated in paints, paper, toothpaste, and plastics. It is aswell acclimated in cement, in gemstones, as an optical opacifier in paper, and a deepening abettor in graphite blended fishing rods and golf clubs.
TiO2 crumb is chemically inert, resists crumbling in sunlight, and is actual opaque: this allows it to admit a authentic and ablaze white blush to the amber or gray chemicals that anatomy the majority of domiciliary plastics. In nature, this admixture is begin in the minerals anatase, brookite, and rutile. Paint fabricated with titanium dioxide does able-bodied in astringent temperatures, and stands up to abyssal environments. Authentic titanium dioxide has a actual top basis of refraction and an optical burning college than diamond. In accession to getting a actual important pigment, titanium dioxide is aswell acclimated in sunscreens due to its adeptness to assure derma by itself. Recently, titanium oxide has been put to use in air purifiers (as a clarify coating), or in blur acclimated to covering windows on barrio so that if titanium oxide becomes apparent to UV ablaze (either solar or artificial) and damp in the air, acknowledging redox breed like hydroxyl radicals are produced so that they can absolve the air or accumulate window surfaces clean.
Aerospace and marine
Due to their top compactness backbone to physique ratio,high bane resistance, fatigue resistance, top able resistance, and adeptness to bear moderately top temperatures after creeping, titanium alloys are acclimated in aircraft, armor plating, argosy ships, spacecraft, and missiles. For these applications titanium adulterated with aluminium, vanadium, and added elements is acclimated for a array of apparatus including analytical structural parts, blaze walls, landing gear, bankrupt ducts (helicopters), and hydraulic systems. In fact, about two thirds of all titanium metal produced is acclimated in aircraft engines and frames. The SR-71 "Blackbird" was one of the aboriginal aircraft to accomplish all-encompassing use of titanium aural its structure, paving the way for its use in avant-garde aggressive and bartering aircraft. An estimated 59 metric bags (130,000 pounds) are acclimated in the Boeing 777, 45 in the Boeing 747, 18 in the Boeing 737, 32 in the Airbus A340, 18 in the Airbus A330, and 12 in the Airbus A320. The Airbus A380 may use 146 metric tons, including about 26 bags in the engines. In engine applications, titanium is acclimated for rotors, compressor blades, hydraulic arrangement components, and nacelles. The titanium 6AL-4V admixture accounts for about 50% of all alloys acclimated in aircraft applications.
Due to its top bane attrition to sea water, titanium is acclimated to accomplish ballista shafts and accouterment and in the calefaction exchangers of desalination plants; in heater-chillers for alkali baptize aquariums, fishing band and leader, and for divers' knives. Titanium is acclimated to accomplish the housings and added apparatus of ocean-deployed surveillance and ecology accessories for accurate and aggressive use. The above Soviet Union developed techniques for authoritative submarines abundantly out of titanium.
Industrial
Welded Titanium aqueduct and action accessories (heat exchangers, tanks, action vessels, valves) are acclimated in the actinic and petrochemical industries primarily for bane resistance. Specific alloys are acclimated in downhole and nickel hydrometallurgy applications due to their top backbone (e. g.: titanium Beta C alloy), bane resistance, or aggregate of both. The lurid and cardboard industry uses titanium in action accessories apparent to acerb media such as sodium hypochlorite or wet chlorine gas (in the bleachery). Added applications include: accelerated welding, beachcomber soldering, and sputtering targets.
Titanium tetrachloride (TiCl4), a achromatic liquid, is important as an average in the action of authoritative TiO2 and is aswell acclimated to aftermath the Ziegler-Natta catalyst, and is acclimated to iridize bottle and because it effluvium acerb in clammy air it is aswell acclimated to accomplish smoke screens.
Medical
Because it is biocompatible (non-toxic and is not alone by the body), titanium is acclimated in a area of medical applications including surgical accouterments and implants, such as hip assurance and sockets (joint replacement) that can break in abode for up to 20 years. The titanium is generally adulterated with about 4% aluminium or 6% Al and 4% vanadium.
Titanium has the inherent acreage to osseointegrate, enabling use in dental implants that can abide in abode for over 30 years. This acreage is aswell advantageous for orthopedic implant applications.[29] These account from titanium's lower modulus of animation (Young's modulus) to added carefully bout that of the cartilage that such accessories are advised to repair. As a result, ashen endless are added analogously aggregate amid cartilage and implant, arch to a lower accident of cartilage abasement due to accent careful and periprosthetic cartilage fractures which action at the boundaries of orthopedic implants. However, titanium alloys' acerbity is still added than alert that of cartilage so adjoining cartilage bears a abundantly bargain amount and may deteriorate.
Since titanium is non-ferromagnetic, patients with titanium implants can be cautiously advised with alluring resonance imaging (convenient for abiding implants). Preparing titanium for article in the physique involves subjecting it to a high-temperature claret arc which removes the apparent atoms, advertisement beginning titanium that is instantly oxidized.
Titanium is aswell acclimated for the surgical instruments acclimated in image-guided surgery, as able-bodied as wheelchairs, crutches, and any added articles area top backbone and low weight are desirable.
More about: Titanium sale
Read more: metal oxide
Titanium was apparent in Cornwall, Great Britain, by William Gregor in 1791 and called by Martin Heinrich Klaproth for the Titans of Greek mythology. The aspect occurs aural a amount of mineral deposits, principally rutile and ilmenite, which are broadly broadcast in the Earth's band and lithosphere, and it is begin in about all active things, rocks, baptize bodies, and soils. The metal is extracted from its arch mineral ores via the Kroll action or the Hunter process. Its a lot of accepted compound, titanium dioxide, is a accepted photocatalyst and is acclimated in the accomplish of white pigments. Added compounds cover titanium tetrachloride (TiCl4), a basic of smoke screens and catalysts; and titanium trichloride (TiCl3), which is acclimated as a agitator in the assembly of polypropylene.
Titanium can be adulterated with iron, aluminium, vanadium, molybdenum, a part of added elements, to aftermath able failing alloys for aerospace (jet engines, missiles, and spacecraft), military, automated action (chemicals and petro-chemicals, desalination plants, pulp, and paper), automotive, agri-food, medical prostheses, orthopedic implants, dental and endodontic instruments and files, dental implants, antic goods, jewelry, adaptable phones, and added applications.
The two a lot of advantageous backdrop of the metal anatomy are bane attrition and the accomplished strength-to-weight arrangement of any metal. In its 18-carat condition, titanium is as able as some steels, but 45% lighter. There are two allotropic forms and 5 by itself occurring isotopes of this element, 46Ti through 50Ti, with 48Ti getting the a lot of abounding (73.8%). Titanium's backdrop are chemically and physically agnate to zirconium, because both of them accept the aforementioned amount of valence electrons and are in the aforementioned accumulation in the alternate table.
Applications
Titanium is acclimated in animate as an alloying aspect (ferro-titanium) to abate atom admeasurement and as a deoxidizer, and in stainless animate to abate carbon content. Titanium is generally adulterated with aluminium (to clarify atom size), vanadium, chestnut (to harden), iron, manganese, molybdenum, and with added metals. Applications for titanium comminute articles (sheet, plate, bar, wire, forgings, castings) can be begin in industrial, aerospace, recreational, and arising markets. Powdered titanium is acclimated in pyrotechnics as a antecedent of bright-burning particles.
Pigments, additives and coatings
About 95% of titanium ore extracted from the Earth is destined for clarification into titanium dioxide (TiO2), an acutely white abiding colorant acclimated in paints, paper, toothpaste, and plastics. It is aswell acclimated in cement, in gemstones, as an optical opacifier in paper, and a deepening abettor in graphite blended fishing rods and golf clubs.
TiO2 crumb is chemically inert, resists crumbling in sunlight, and is actual opaque: this allows it to admit a authentic and ablaze white blush to the amber or gray chemicals that anatomy the majority of domiciliary plastics. In nature, this admixture is begin in the minerals anatase, brookite, and rutile. Paint fabricated with titanium dioxide does able-bodied in astringent temperatures, and stands up to abyssal environments. Authentic titanium dioxide has a actual top basis of refraction and an optical burning college than diamond. In accession to getting a actual important pigment, titanium dioxide is aswell acclimated in sunscreens due to its adeptness to assure derma by itself. Recently, titanium oxide has been put to use in air purifiers (as a clarify coating), or in blur acclimated to covering windows on barrio so that if titanium oxide becomes apparent to UV ablaze (either solar or artificial) and damp in the air, acknowledging redox breed like hydroxyl radicals are produced so that they can absolve the air or accumulate window surfaces clean.
Aerospace and marine
Due to their top compactness backbone to physique ratio,high bane resistance, fatigue resistance, top able resistance, and adeptness to bear moderately top temperatures after creeping, titanium alloys are acclimated in aircraft, armor plating, argosy ships, spacecraft, and missiles. For these applications titanium adulterated with aluminium, vanadium, and added elements is acclimated for a array of apparatus including analytical structural parts, blaze walls, landing gear, bankrupt ducts (helicopters), and hydraulic systems. In fact, about two thirds of all titanium metal produced is acclimated in aircraft engines and frames. The SR-71 "Blackbird" was one of the aboriginal aircraft to accomplish all-encompassing use of titanium aural its structure, paving the way for its use in avant-garde aggressive and bartering aircraft. An estimated 59 metric bags (130,000 pounds) are acclimated in the Boeing 777, 45 in the Boeing 747, 18 in the Boeing 737, 32 in the Airbus A340, 18 in the Airbus A330, and 12 in the Airbus A320. The Airbus A380 may use 146 metric tons, including about 26 bags in the engines. In engine applications, titanium is acclimated for rotors, compressor blades, hydraulic arrangement components, and nacelles. The titanium 6AL-4V admixture accounts for about 50% of all alloys acclimated in aircraft applications.
Due to its top bane attrition to sea water, titanium is acclimated to accomplish ballista shafts and accouterment and in the calefaction exchangers of desalination plants; in heater-chillers for alkali baptize aquariums, fishing band and leader, and for divers' knives. Titanium is acclimated to accomplish the housings and added apparatus of ocean-deployed surveillance and ecology accessories for accurate and aggressive use. The above Soviet Union developed techniques for authoritative submarines abundantly out of titanium.
Industrial
Welded Titanium aqueduct and action accessories (heat exchangers, tanks, action vessels, valves) are acclimated in the actinic and petrochemical industries primarily for bane resistance. Specific alloys are acclimated in downhole and nickel hydrometallurgy applications due to their top backbone (e. g.: titanium Beta C alloy), bane resistance, or aggregate of both. The lurid and cardboard industry uses titanium in action accessories apparent to acerb media such as sodium hypochlorite or wet chlorine gas (in the bleachery). Added applications include: accelerated welding, beachcomber soldering, and sputtering targets.
Titanium tetrachloride (TiCl4), a achromatic liquid, is important as an average in the action of authoritative TiO2 and is aswell acclimated to aftermath the Ziegler-Natta catalyst, and is acclimated to iridize bottle and because it effluvium acerb in clammy air it is aswell acclimated to accomplish smoke screens.
Medical
Because it is biocompatible (non-toxic and is not alone by the body), titanium is acclimated in a area of medical applications including surgical accouterments and implants, such as hip assurance and sockets (joint replacement) that can break in abode for up to 20 years. The titanium is generally adulterated with about 4% aluminium or 6% Al and 4% vanadium.
Titanium has the inherent acreage to osseointegrate, enabling use in dental implants that can abide in abode for over 30 years. This acreage is aswell advantageous for orthopedic implant applications.[29] These account from titanium's lower modulus of animation (Young's modulus) to added carefully bout that of the cartilage that such accessories are advised to repair. As a result, ashen endless are added analogously aggregate amid cartilage and implant, arch to a lower accident of cartilage abasement due to accent careful and periprosthetic cartilage fractures which action at the boundaries of orthopedic implants. However, titanium alloys' acerbity is still added than alert that of cartilage so adjoining cartilage bears a abundantly bargain amount and may deteriorate.
Since titanium is non-ferromagnetic, patients with titanium implants can be cautiously advised with alluring resonance imaging (convenient for abiding implants). Preparing titanium for article in the physique involves subjecting it to a high-temperature claret arc which removes the apparent atoms, advertisement beginning titanium that is instantly oxidized.
Titanium is aswell acclimated for the surgical instruments acclimated in image-guided surgery, as able-bodied as wheelchairs, crutches, and any added articles area top backbone and low weight are desirable.
More about: Titanium sale
Read more: metal oxide
Thursday, 8 March 2012
What is CADMIUM CARBONATE used for?
CADMIUM CARBONATE
Trade Name: Cadmium Carbonate
Chemical Family: Metal carbonate
Formula: CdCO3 CAS : 513-78-0
Boiling Point: N/E or N/A
Melting Point: Not Applicable 4.258 at 4.0 oC (H2O=1)
Vapor Density: N/A
Specific Gravity:
Solubility in H2O: Insoluble, soluble in acids, KCN, NH4 salts
Volatiles by Weight: N/A
Appearance and Odor: White, amorphous powder, no odor.
Descriptions of CADMIUM CARBONATE
Used for the accomplish polyester intermediates, bottle colorant alteration and amoebic reactions catalyst, artificial plasticizer, balance and assembly cadmium materials.
More about: CADMIUM CARBONATE sale
Read more: metal oxide metal
Trade Name: Cadmium Carbonate
Chemical Family: Metal carbonate
Formula: CdCO3 CAS : 513-78-0
Boiling Point: N/E or N/A
Melting Point: Not Applicable 4.258 at 4.0 oC (H2O=1)
Vapor Density: N/A
Specific Gravity:
Solubility in H2O: Insoluble, soluble in acids, KCN, NH4 salts
Volatiles by Weight: N/A
Appearance and Odor: White, amorphous powder, no odor.
Descriptions of CADMIUM CARBONATE
Used for the accomplish polyester intermediates, bottle colorant alteration and amoebic reactions catalyst, artificial plasticizer, balance and assembly cadmium materials.
More about: CADMIUM CARBONATE sale
Read more: metal oxide metal
Wednesday, 7 March 2012
Uses of Cadmium oxide
Cadmium oxide is an comatose admixture with the adapt CdO. It is one of the basic precursors to added cadmium compounds. It crystallizes in a cubic rocksalt applique like sodium chloride, with octahedral cation and anion centers. It occurs by itself as the abate mineral monteponite. Cadmium oxide can be activate as a achromatic billowing atom or as amber or red crystals. Cadmium oxide is an n-type semiconductor with a cast gap of 2.16 eV at allowance temperature.
Since cadmium compounds are about activate in amalgamation with zinc ores, cadmium oxide is a accustomed by-product of zinc refining.[9] It is produced by afire basal cadmium in air. Pyrolysis of added cadmium compounds, such as the nitrate or the carbonate, aswell affords this oxide. If pure, it is red but CdO is aberrant in accepting attainable in abounding differing colours due to its addiction to analysis birthmark structures constant from anion vacancies. Cadmium oxide is able commercially by acerbic cadmium animation in air.
Uses
Cadmium oxide is acclimated in cadmium plating baths, electrodes for accumulator batteries, cadmium salts, catalyst, bowl glazes, phosphors, and nematocide. Major uses for cadmium oxide are as an additive for electroplating baths, and in pigments.
CdO is acclimated as a cellophane conductive material, which was able as a cellophane administering blur aback in 1907. Cadmium oxide in the anatomy of attenuate films has been acclimated in applications such as photodiodes, phototransistors, photovoltaic cells, cellophane electrodes, aqueous clear displays, IR detectors, and anti absorption coatings. CdO microparticles abide bandgap action if apparent to UV-A ablaze and is aswell careful in phenol photodegradation.
Cadmium plating
Most bartering electroplating of cadmium is done by electrodeposition from cyanide baths. These cyanide baths abide of cadmium oxide and sodium cyanide in water, which acceptable anatomy cadmium cyanide and sodium hydroxide. A archetypal blueprint is 32 g/L cadmium oxide and 75 g/L sodium cyanide. The cadmium absorption may alter by as abundant as 50%. Brighteners are usually added to the ablution and the plating is done at allowance temperature with top abstention cadmium anodes.
More about: Cadmium oxide sale
Read more: Cobalt oxide
Since cadmium compounds are about activate in amalgamation with zinc ores, cadmium oxide is a accustomed by-product of zinc refining.[9] It is produced by afire basal cadmium in air. Pyrolysis of added cadmium compounds, such as the nitrate or the carbonate, aswell affords this oxide. If pure, it is red but CdO is aberrant in accepting attainable in abounding differing colours due to its addiction to analysis birthmark structures constant from anion vacancies. Cadmium oxide is able commercially by acerbic cadmium animation in air.
Uses
Cadmium oxide is acclimated in cadmium plating baths, electrodes for accumulator batteries, cadmium salts, catalyst, bowl glazes, phosphors, and nematocide. Major uses for cadmium oxide are as an additive for electroplating baths, and in pigments.
CdO is acclimated as a cellophane conductive material, which was able as a cellophane administering blur aback in 1907. Cadmium oxide in the anatomy of attenuate films has been acclimated in applications such as photodiodes, phototransistors, photovoltaic cells, cellophane electrodes, aqueous clear displays, IR detectors, and anti absorption coatings. CdO microparticles abide bandgap action if apparent to UV-A ablaze and is aswell careful in phenol photodegradation.
Cadmium plating
Most bartering electroplating of cadmium is done by electrodeposition from cyanide baths. These cyanide baths abide of cadmium oxide and sodium cyanide in water, which acceptable anatomy cadmium cyanide and sodium hydroxide. A archetypal blueprint is 32 g/L cadmium oxide and 75 g/L sodium cyanide. The cadmium absorption may alter by as abundant as 50%. Brighteners are usually added to the ablution and the plating is done at allowance temperature with top abstention cadmium anodes.
More about: Cadmium oxide sale
Read more: Cobalt oxide
Uses of Cadmium chloride
Cadmium chloride is a white apparent admixture of cadmium and chlorine, with the blueprint CdCl2. It is a hygroscopic solid that is awful acrid in baptize and hardly acrid in alcohol. Although it is advised to be ionic, it has ample covalent appearance to its bonding. The clear anatomy of cadmium chloride (described below), composed of two-dimensional layers of ions, is a advertence for anecdotic added clear structures. Aswell accepted are CdCl2.H2O and CdCl2.5H2O.
Structure
Cadmium chloride forms crystals with rhombohedral symmetry. Cadmium iodide, CdI2, has a actual agnate clear anatomy to CdCl2. The alone layers in the two structures are identical, but in CdCl2 the chloride ions are abiding in a CCP lattice, admitting in CdI2 the iodide ions are abiding in a HCP lattice.
Uses
Cadmium chloride is acclimated for the alertness of cadmium sulfide, acclimated as "Cadmium Yellow", a brilliant-yellow abiding asleep pigment.
CdCl2 + H2S → CdS + 2 HCl
In the laboratory, anhydrous CdCl2 can be acclimated for the alertness of organocadmium compounds of the blazon R2Cd, area R is an aryl or a primary alkyl. These were already acclimated in the amalgam of ketones from acyl chlorides:
CdCl2 + 2 RMgX → R2Cd + MgCl2 + MgX2
R2Cd + R'COCl → R'COR + CdCl2
Such reagents accept abundantly been supplanted by organocopper compounds, which are abundant beneath toxic.
Cadmium chloride is aswell acclimated for photocopying, dyeing and electroplating.
More about: Cadmium chloride sale
Read more: metal oxide
Structure
Cadmium chloride forms crystals with rhombohedral symmetry. Cadmium iodide, CdI2, has a actual agnate clear anatomy to CdCl2. The alone layers in the two structures are identical, but in CdCl2 the chloride ions are abiding in a CCP lattice, admitting in CdI2 the iodide ions are abiding in a HCP lattice.
Uses
Cadmium chloride is acclimated for the alertness of cadmium sulfide, acclimated as "Cadmium Yellow", a brilliant-yellow abiding asleep pigment.
CdCl2 + H2S → CdS + 2 HCl
In the laboratory, anhydrous CdCl2 can be acclimated for the alertness of organocadmium compounds of the blazon R2Cd, area R is an aryl or a primary alkyl. These were already acclimated in the amalgam of ketones from acyl chlorides:
CdCl2 + 2 RMgX → R2Cd + MgCl2 + MgX2
R2Cd + R'COCl → R'COR + CdCl2
Such reagents accept abundantly been supplanted by organocopper compounds, which are abundant beneath toxic.
Cadmium chloride is aswell acclimated for photocopying, dyeing and electroplating.
More about: Cadmium chloride sale
Read more: metal oxide
Monday, 5 March 2012
Applications of Cadmium sulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications.
Cadmium sulfide can be prepared by the precipitation from soluble cadmium(II) salts with sulfide ion and this has been used in the past for gravimetric analysis and qualitative inorganic analysis.
Pigment production usually involves the precipitation of CdS, the washing of the precipitate to remove soluble cadmium salts followed by calcination (roasting) to convert it to the hexagonal form followed by milling to produce a powder. When cadmium sulfide selenides are required the CdSe is co-precipitated with CdS and the cadmium sulfoselenide is created during the calcination step.
Properties
Cadmium sulfide has, like zinc sulfide, two crystal forms; the more stable hexagonal wurtzite structure (found in the mineral Greenockite) and the cubic zinc blende structure (found in the mineral Hawleyite). In both of these forms the cadmium and sulfur atoms are four coordinate. There is also a high pressure form with the NaCl rock salt structure.
Cadmium sulfide is a direct band gap semiconductor. The magnitude of its band gap means that it appears coloured.
As well as this obvious property others properties result:
the conductivity increases when irradiated with light(leading to uses as a photoresistor)
when combined with a p-type semiconductor it forms the core component of a photovoltaic (solar) cell and a CdS/Cu2S solar cell was one of the first efficient cells to be reported (1954)
when doped with for example Cu+ ("activator") and Al3+ ("coactivator") CdS luminesces under electron beam excitation (cathodoluminescence) and is used as phosphor
both polymorphs are piezoelectric and the hexagonal is also pyroelectric
electroluminescence
CdS crystal can act as a solid state laser
Applications
CdS is mainly used as a pigment.
CdS and cadmium selenide are used in manufacturing of photoresistors (light dependent resistors) sensitive to visible and near infrared light.
In thin-film form, CdS can be combined with other layers for use in certain types of solar cells. CdS was also one of the first semiconductor materials to be used for thin-film transistors (TFTs). However interest in compound semiconductors for TFTs largely waned after the emergence of amorphous silicon technology in the late 1970s.
Pigment
Cadmium sulfide is known as cadmium yellow (CI pigment yellow 37). By adding varying amounts of selenium as selenide, it is possible to obtain a range of colors, for example CI pigment orange 20 and CI pigment red 108.
Synthetic cadmium pigments based on cadmium sulfide are valued for their good thermal stability, light and weather fastness, chemical resistance and high opacity. The general commercial availability of cadmium sulfide from the 1840s led to its adoption by artists, notably Van Gogh, Monet (in his London series and other works) and Matisse (Bathers by a river 1916–1919). The presence of cadmium in paints has been used to detect forgeries in paintings alleged to have been produced prior to the 19th century. CdS is used as pigment in plastics
More about: Cadmium sulfide sale
Read more: Cobalt oxide
Cadmium sulfide can be prepared by the precipitation from soluble cadmium(II) salts with sulfide ion and this has been used in the past for gravimetric analysis and qualitative inorganic analysis.
Pigment production usually involves the precipitation of CdS, the washing of the precipitate to remove soluble cadmium salts followed by calcination (roasting) to convert it to the hexagonal form followed by milling to produce a powder. When cadmium sulfide selenides are required the CdSe is co-precipitated with CdS and the cadmium sulfoselenide is created during the calcination step.
Properties
Cadmium sulfide has, like zinc sulfide, two crystal forms; the more stable hexagonal wurtzite structure (found in the mineral Greenockite) and the cubic zinc blende structure (found in the mineral Hawleyite). In both of these forms the cadmium and sulfur atoms are four coordinate. There is also a high pressure form with the NaCl rock salt structure.
Cadmium sulfide is a direct band gap semiconductor. The magnitude of its band gap means that it appears coloured.
As well as this obvious property others properties result:
the conductivity increases when irradiated with light(leading to uses as a photoresistor)
when combined with a p-type semiconductor it forms the core component of a photovoltaic (solar) cell and a CdS/Cu2S solar cell was one of the first efficient cells to be reported (1954)
when doped with for example Cu+ ("activator") and Al3+ ("coactivator") CdS luminesces under electron beam excitation (cathodoluminescence) and is used as phosphor
both polymorphs are piezoelectric and the hexagonal is also pyroelectric
electroluminescence
CdS crystal can act as a solid state laser
Applications
CdS is mainly used as a pigment.
CdS and cadmium selenide are used in manufacturing of photoresistors (light dependent resistors) sensitive to visible and near infrared light.
In thin-film form, CdS can be combined with other layers for use in certain types of solar cells. CdS was also one of the first semiconductor materials to be used for thin-film transistors (TFTs). However interest in compound semiconductors for TFTs largely waned after the emergence of amorphous silicon technology in the late 1970s.
Pigment
Cadmium sulfide is known as cadmium yellow (CI pigment yellow 37). By adding varying amounts of selenium as selenide, it is possible to obtain a range of colors, for example CI pigment orange 20 and CI pigment red 108.
Synthetic cadmium pigments based on cadmium sulfide are valued for their good thermal stability, light and weather fastness, chemical resistance and high opacity. The general commercial availability of cadmium sulfide from the 1840s led to its adoption by artists, notably Van Gogh, Monet (in his London series and other works) and Matisse (Bathers by a river 1916–1919). The presence of cadmium in paints has been used to detect forgeries in paintings alleged to have been produced prior to the 19th century. CdS is used as pigment in plastics
More about: Cadmium sulfide sale
Read more: Cobalt oxide
Sunday, 4 March 2012
Applications of Potassium carbonate
Potassium carbonate (K2CO3) is a white salt, soluble in water (insoluble in alcohol), which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid. Potassium carbonate is used in the production of soap and glass.
Potassium carbonate was first identified in 1742 by Antonio Campanella and is the primary component of [potash] and the more refined pearl ash or salts of tartar. Historically pearl ash was created by baking potash in a kiln to remove impurities. The fine white powder remaining was the pearl ash. The first patent issued by the U.S. Patent Office was awarded to Samuel Hopkins in 1790 for an improved method of making potash and pearl ash.
In late 18th century North America, before the development of baking powder, pearl ash was used as a leavening agent in quick breads.
Applications of Potassium carbonate
Pearl ash has been used for soap, glass, and china production.
Pearl ash added to hard water will soften the water.
In the laboratory, it may be used as a mild drying agent where other drying agents such as calcium chloride and magnesium sulfate may be incompatible. However, it is not suitable for acidic compounds, but can be useful for drying an organic phase if one has a small amount of acidic impurity.
Mixed with water it causes an exothermic reaction.
It is mixed with distilled water to make a safer electrolyte for oxyhydrogen production than potassium hydroxide, the more commonly used electrolyte.
In cuisine, it is used as an ingredient in the production of grass jelly, a food consumed in Chinese and Southeast Asian cuisines. It is used to tenderize tripe. German gingerbread recipes often use potassium carbonate as a baking agent.
Potassium carbonate is being used as the electrolyte in many cold fusion experiments.
Potassium carbonate is sometimes used as a buffering agent in the production of mead or wine.
Aqueous potassium carbonate is used in the fertilizer industry for removal of carbon dioxide from the ammonia production synthesis gas coming from the steam reformer.
Aqueous potassium carbonate is also used as a fire suppressant in extinguishing deep fat fryers and various other B class related fires, and in condensed aerosol fire suppression although as the by-product of potassium nitrate.
Potassium carbonate is used in reactions to maintain anhydrous conditions without reacting with the reactants and product formed.[citation needed] It may also be used to pre-dry some ketones, alcohols, and amines prior to distillation.
An ingredient in welding fluxes, and in the flux coating on arc welding rods.
More about: Potassium carbonate sale
Read more: metal oxide
Potassium carbonate was first identified in 1742 by Antonio Campanella and is the primary component of [potash] and the more refined pearl ash or salts of tartar. Historically pearl ash was created by baking potash in a kiln to remove impurities. The fine white powder remaining was the pearl ash. The first patent issued by the U.S. Patent Office was awarded to Samuel Hopkins in 1790 for an improved method of making potash and pearl ash.
In late 18th century North America, before the development of baking powder, pearl ash was used as a leavening agent in quick breads.
Applications of Potassium carbonate
Pearl ash has been used for soap, glass, and china production.
Pearl ash added to hard water will soften the water.
In the laboratory, it may be used as a mild drying agent where other drying agents such as calcium chloride and magnesium sulfate may be incompatible. However, it is not suitable for acidic compounds, but can be useful for drying an organic phase if one has a small amount of acidic impurity.
Mixed with water it causes an exothermic reaction.
It is mixed with distilled water to make a safer electrolyte for oxyhydrogen production than potassium hydroxide, the more commonly used electrolyte.
In cuisine, it is used as an ingredient in the production of grass jelly, a food consumed in Chinese and Southeast Asian cuisines. It is used to tenderize tripe. German gingerbread recipes often use potassium carbonate as a baking agent.
Potassium carbonate is being used as the electrolyte in many cold fusion experiments.
Potassium carbonate is sometimes used as a buffering agent in the production of mead or wine.
Aqueous potassium carbonate is used in the fertilizer industry for removal of carbon dioxide from the ammonia production synthesis gas coming from the steam reformer.
Aqueous potassium carbonate is also used as a fire suppressant in extinguishing deep fat fryers and various other B class related fires, and in condensed aerosol fire suppression although as the by-product of potassium nitrate.
Potassium carbonate is used in reactions to maintain anhydrous conditions without reacting with the reactants and product formed.[citation needed] It may also be used to pre-dry some ketones, alcohols, and amines prior to distillation.
An ingredient in welding fluxes, and in the flux coating on arc welding rods.
More about: Potassium carbonate sale
Read more: metal oxide
Thursday, 1 March 2012
What is Rubidium carbonate used for?
Rubidium carbonate is a water insoluble Rubidium source that can easily be converted to other Rubidium compounds, such as the oxide by heating (calcination). Carbonate compounds also give off carbon dioxide when treated with dilute acids. Rubidium Carbonate is generally immediately available in most volumes.
Rubidium carbonate, Rb2CO3, is a convenient compound of rubidium; it is stable, not particularly reactive, and readily soluble in water, and is the form in which rubidium is usually sold.
Rubidium carbonate can be prepared by adding ammonium carbonate to rubidium hydroxide.
Uses
Rubidium carbonate is used in some kinds of glass-making by enhancing stability and durability as well as reducing its conductivity. Rubidium carbonate is also used as a part of a catalyst for preparing short-chain alcohols from feed gas. Analytics reagents, also used in synthetic other rb salt.
More about: Rubidium carbonate sale
Read more: metal oxide metal
Rubidium carbonate, Rb2CO3, is a convenient compound of rubidium; it is stable, not particularly reactive, and readily soluble in water, and is the form in which rubidium is usually sold.
Rubidium carbonate can be prepared by adding ammonium carbonate to rubidium hydroxide.
Uses
Rubidium carbonate is used in some kinds of glass-making by enhancing stability and durability as well as reducing its conductivity. Rubidium carbonate is also used as a part of a catalyst for preparing short-chain alcohols from feed gas. Analytics reagents, also used in synthetic other rb salt.
More about: Rubidium carbonate sale
Read more: metal oxide metal
Subscribe to:
Comments (Atom)

+carbonate+hydroxide.gif)